Geographic atrophy progression
Regions of atrophy typically start outside the fovea and expand to involve the fovea, which – over time – leads to permanent loss of vision.1
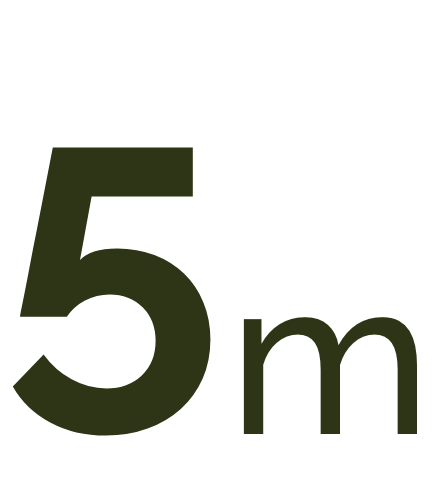
Currently geographic atrophy affects more than 5 million people worldwide. This number is expected to increase to more than 18 million by 2040.1
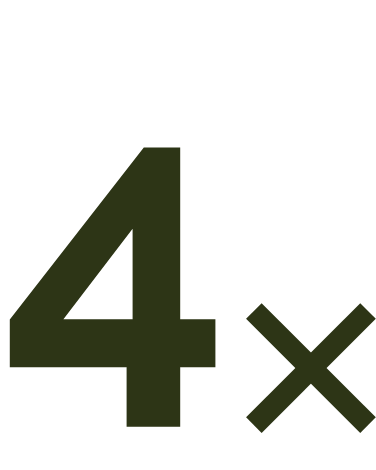
From age 50, prevalence
quadruples every 10 years.6

Geographic atrophy accounts
for up to 20% of all legal blindness attributed to AMD.7,8

An eye with geographic atrophy can also naturally develop wet AMD and vice versa.9
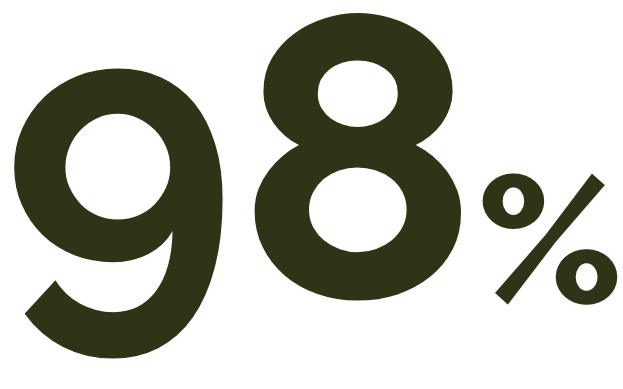
of patients with wet AMD progressed to geographic atrophy over an average of 7.3 years of follow-up.10
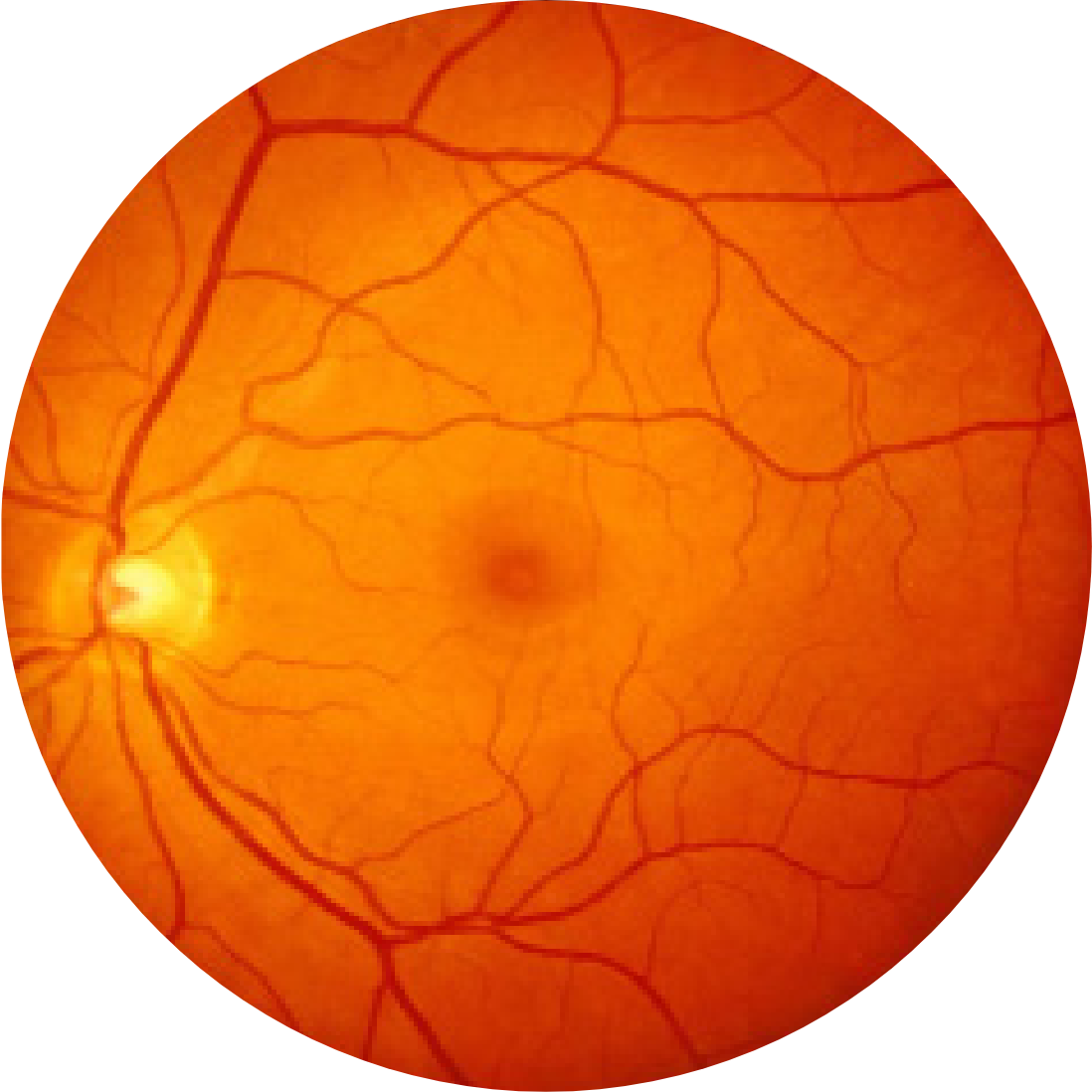
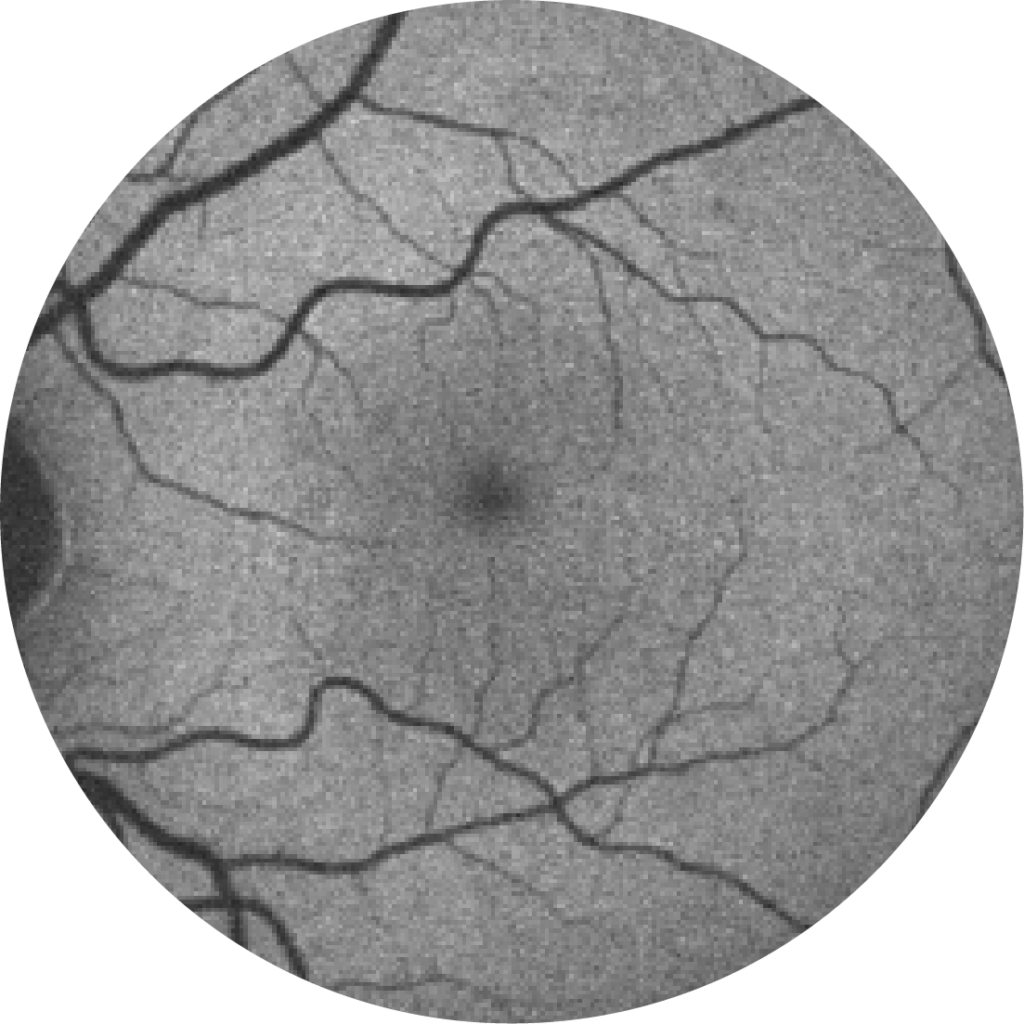
Fundus photograph of a healthy eye
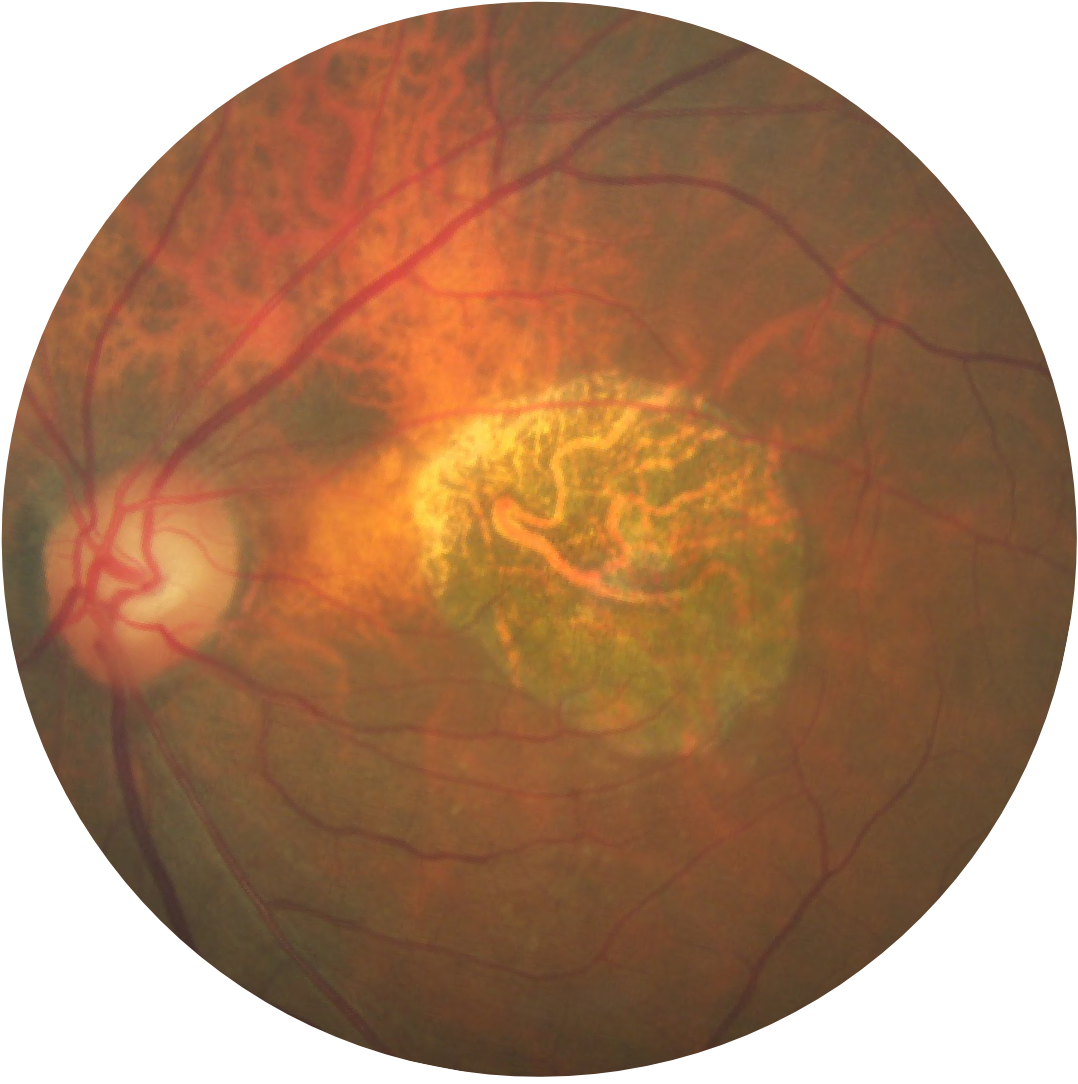
Fundus photograph of an eye with geographic atrophy
Geographic atrophy is characterised by progressive and irreversible loss of the photoreceptors, retinal pigment epithelium (RPE) and underlying choriocapillaris.1,2
Regions of atrophy typically start outside the fovea and expand to involve the fovea, which – over time – leads to permanent loss of vision.1
Causes of geographic atrophy
Age-related macular degeneration is a complex, multifactorial disease and geographic atrophy pathogenesis encompasses a complex interaction of genetic, physiological and environmental factors.11-14
Genetics
- Drusen formation
- Formation of reactive oxygen species
- Inflammation
- Immune response, including complement
Physiology
- Age is the greatest risk factor for geographic atrophy
- Certain types of dyslipidaemias
Environment
- Sunlight, smoking and diet
- High alcohol intake
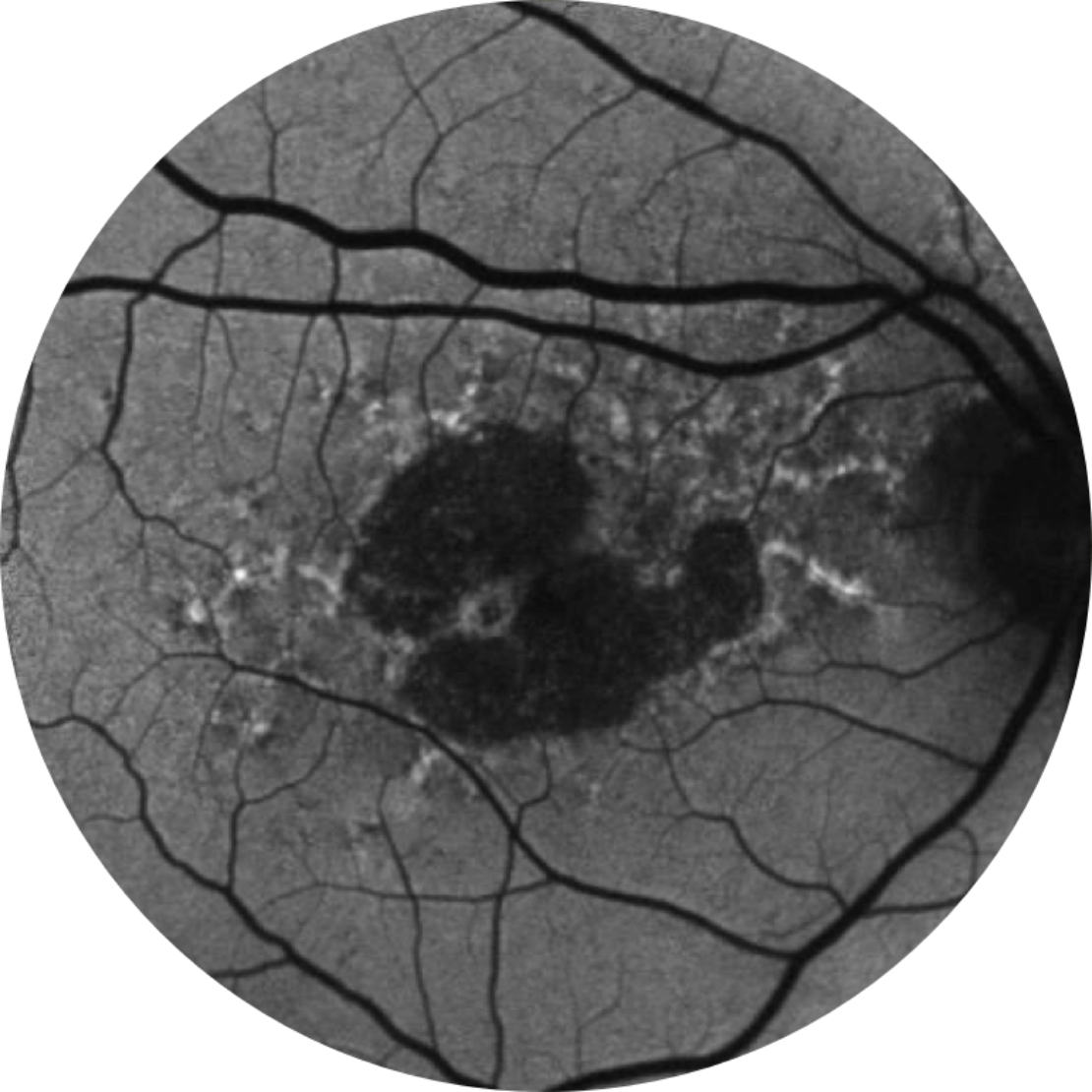
Baseline Year 1
BCVA 20/63+, GA Area 5.18 mm2
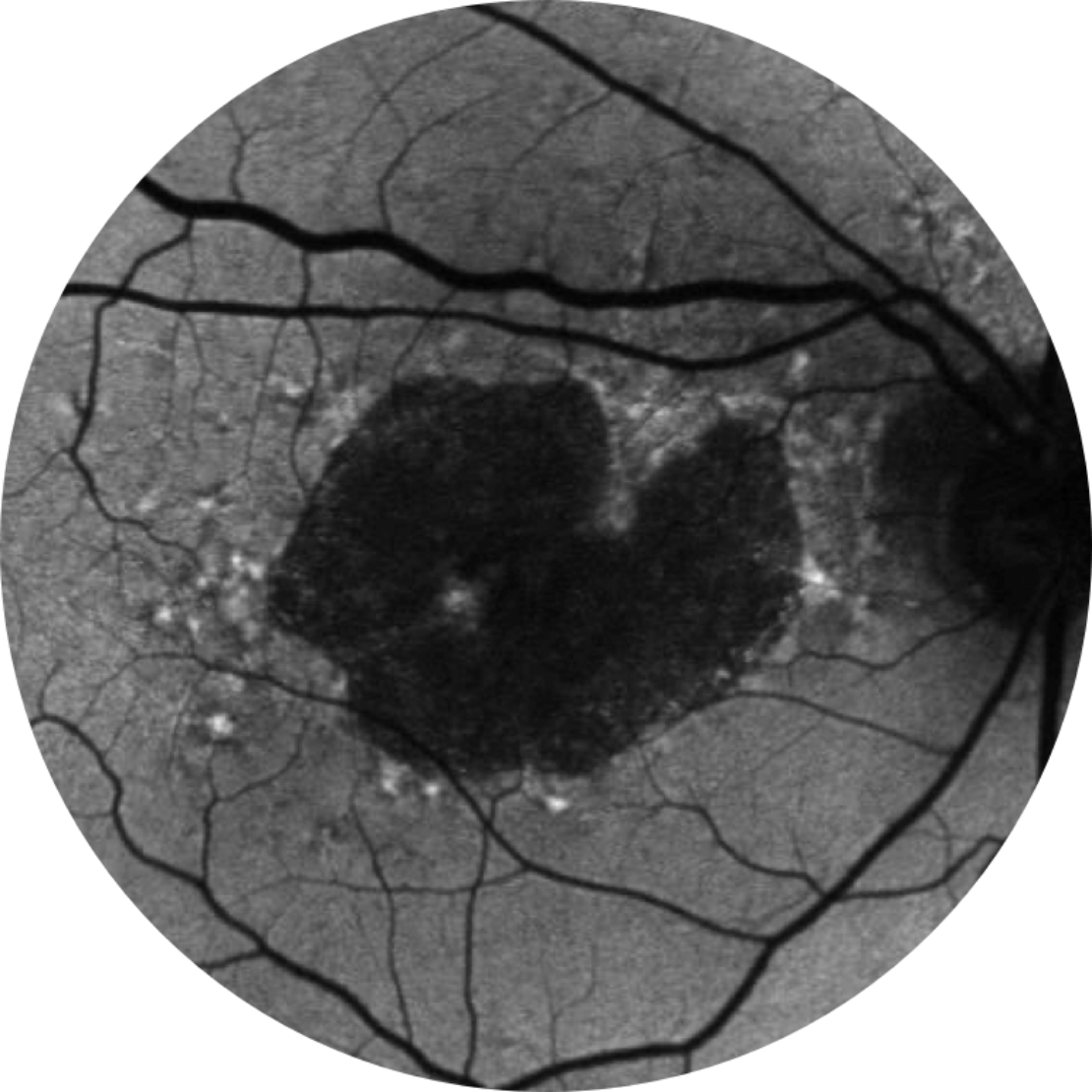
Baseline Year 2
BCVA 20/80-2, GA Area 10.39 mm2
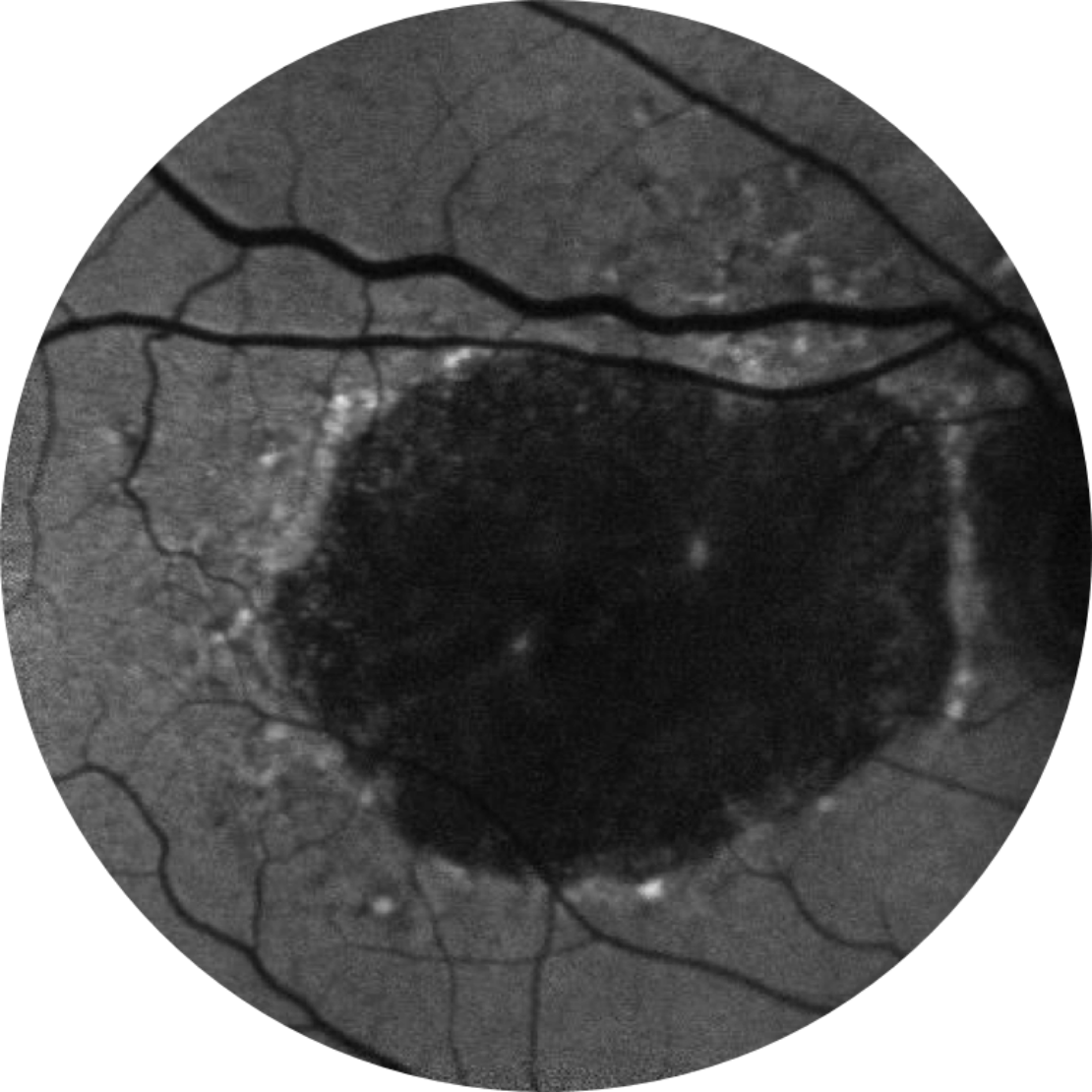
Baseline Year 5
BCVA 20/200, GA Area 18.58 mm2
Lesion growth may lead to visual decline.2,16,17
Visual acuity does not strongly correlate with geographic atophy lesion growth. Functional vision declines as lesions grow.15
BCVA = Best-corrected visual acuity
Diagnosis of geographic atrophy
Retinal imaging techniques are used to identify, diagnose and monitor all stages of age-related macular degeneration, including geographic atrophy.18
When diagnosing and monitoring age-related macular degeneration an ophthalmologist, retinal specialist or optometrist will look for the following features in the retina:19
• Decorated with drusen
• A sharply demarcated area in the macular region with an atrophic retina, lacking pigmentation
• Visible underlying choroidal blood vessels
Normal fundus autofluorescence of a retina
Fundus autofluorescence angiography imaging is currently a standard imaging technology to visualise the retinal pigment epithelium (RPE) in geographic atrophy.20
Horizontal OCT scan over the fovea
Optical coherence tomography (OCT): the atrophy of the retinal layers can be clearly seen with this non-invasive imaging technique.21
Current management approaches
Focus on coping with the disease, without slowing or stopping lesion growth.22-24
VISUAL REHABILITATION25
LOW VISION AIDS26
AREDS SUPPLEMENTS27
SMOKING CESSATION27
EXERCISE28
DIET27
AREDS = Age-Related Eye Disease Study.
AREDS supplements are not an approved therapy for GA.
Explore the role of complement overactivation in geographic atrophy
Multiple complement-mediated pathways may be involved in the pathogenesis of geographic atrophy. Inhibiting the complement pathway is an attractive therapeutic target to slow the progression of GA.29,30
References
- Fleckenstein, M. et al. (2018). The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology, 125(3), 369–390. https://doi.org/10.1016/j.ophtha.2017.08.038.
- Boyer, D.S. et al. (2017). The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina, 37(5), pp.819–835. doi:10.1097/iae.0000000000001392.
- Lindblad, A.S. et al. (2009).Change in Area of Geographic Atrophy in the Age-Related Eye Disease Study. Archives of Ophthalmology, 127(9), p.1168. doi:10.1001/archophthalmol.2009.198.
- Holz, F.G. et al. (2014). Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology, [online] 121(5), pp.1079–1091. doi:10.1016/j.ophtha.2013.11.023.
- Sunness, J.S et al. (2007). The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology, [online] 114(2), pp.271–277. doi:10.1016/j.ophtha.2006.09.016.
- Rudnicka, A.R. et al. (2012). Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology, [online] 119(3), pp.571–580. doi:10.1016/j.ophtha.2011.09.027.
- Gehrs, K.M. et al. (2006). Age-related macular degeneration—emerging pathogenetic and therapeutic concepts. Annals of medicine, [online] 38(7), pp.450–471. doi:10.1080/07853890600946724.
- Biarnés, M. et al. (2011). Update on Geographic Atrophy in Age-Related Macular Degeneration. Optometry and Vision Science, 88(7), pp.881–889. doi:10.1097/opx.0b013e31821988c1.
- BrightFocus Foundation. (2018). Age-Related Macular Degeneration: Facts & Figures. [online] Available at: https://www.brightfocus.org/macular/article/age-related-macular-facts-figures. Accessed Apr. 29, 2021.
- Rofagha, S. et al. (2013). Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON. Ophthalmology, 120(11), pp.2292–2299. doi:10.1016/j.ophtha.2013.03.046.
- Buschini, E. et al. (2015). Recent developments in the management of dry age-related macular degeneration. Clinical Ophthalmology, p.563. doi:10.2147/opth.s59724.
- Risk factors associated with age-related macular degeneration. (2000). Ophthalmology, 107(12), pp.2224–2232. doi:10.1016/s0161-6420(00)00409-7.
- Fritsche, L.G. et al. (2016). A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nature genetics, [online] 48(2), pp.134–143. doi:10.1038/ng.3448.
- Chong, E.W. et al. (2008). Alcohol Consumption and the Risk of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. American Journal of Ophthalmology, [online] 145(4), pp.707-715.e2. doi:10.1016/j.ajo.2007.12.005.
- Heier, J.S. et al. (2020). Visual Function Decline Resulting from Geographic Atrophy: Results from the Chroma and Spectri Phase 3 Trials. Ophthalmology. Retina, [online] 4(7), pp.673–688. doi:10.1016/j.oret.2020.01.019.
- Kimel, M. et al. (2017). Functional Reading Independence (FRI) Index: A New Patient-Reported Outcome Measure for Patients With Geographic Atrophy. Investigative Opthalmology & Visual Science, 57(14), p.6298. doi:10.1167/iovs.16-20361.
- Sadda, S.R. Clinical endpoints for the study of geographic atrophy secondary to age-related macular degeneration. Retina, 36(10), pp.1806–1822. doi:10.1097/iae.0000000000001283.
- What is the difference between direct and indirect ophthalmoscopy (2016). What is the difference between direct and indirect ophthalmoscopy? [online] American Academy of Ophthalmology. Available at: https://www.aao.org/eye-health/ask-ophthalmologist-q/what-is-difference-between-direct-indirect-ophthal. Accessed July. 20, 2022.
- eyewiki.aao.org. (n.d.). Geographic Atrophy – EyeWiki. [online] Available at: https://eyewiki.aao.org/Geographic_Atrophy. Accessed July 20, 2022.
- Townsend, W.D. (1992). Scleral depression. Optometry Clinics: The Official Publication of the Prentice Society, [online] 2(3), pp.127–144. Available at: https://pubmed.ncbi.nlm.nih.gov/1463913.
- Ferguson, L.R. et al. (2014). Retinal Thickness Measurement Obtained with Spectral Domain Optical Coherence Tomography Assisted Optical Biopsy Accurately Correlates with Ex Vivo Histology. PLoS ONE, 9(10), p.e111203. doi:10.1371/journal.pone.0111203.
- Bandello, F. et al. (2017). Recent advances in the management of dry age-related macular degeneration: A review. F1000Research, 6, p.245. doi:10.12688/f1000research.10664.1.
- Chakravarthy, U. et al. (2018). Characterizing Disease Burden and Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology, 125(6), pp.842–849. doi:10.1016/j.ophtha.2017.11.036.
- Sacconi, R. et al. (2017). A Review of Current and Future Management of Geographic Atrophy. Ophthalmology and Therapy, [online] 6(1), pp.69–77. doi:10.1007/s40123-017-0086-6.
- Ramírez Estudillo, J.A. et al. (2017). Visual rehabilitation via microperimetry in patients with geographic atrophy: a pilot study. International Journal of Retina and Vitreous, [online] 3, p.21. doi:10.1186/s40942-017-0071-1.
- Gopalakrishnan, S. et al. (2020). Low-vision intervention in individuals with age-related macular degeneration. Indian Journal of Ophthalmology, [online] 68(5), pp.886–889. doi:10.4103/ijo.IJO_1093_19.
- Thorell, M.R. and Rosenfeld, P.J. (2014). Treatment of Geographic Atrophy: What’s on the Horizon? Current Ophthalmology Reports, 2(1), pp.20–25.
doi:10.1007/s40135-013-0036-y. - Seddon, J.M. (2003). Progression of Age-Related Macular Degeneration. Archives of Ophthalmology, 121(6), p.785. doi:10.1001/archopht.121.6.785.
- Katschke, K.J. et al. (2018). Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Scientific Reports, [online] 8(1), p.7348. doi:10.1038/s41598-018-25557-8.
- Heesterbeek, T.J. et al. (2020). Complement Activation Levels Are Related to Disease Stage in AMD. Investigative Ophthalmology & Visual Science, [online] 61(3), pp.18–18. doi:10.1167/iovs.61.3.18.